FREQUENTLY ASKED QUESTIONS
Who is GPH vaccinating?
We have previously been vaccinating group 1a and patients 50+ and are looking to vaccinate other populations shortly.
Governor Janet Mills has announced that all Maine residents aged 18 and over now qualify for vaccination, and beginning April 19, all Mainers 16 and over will qualify for vaccination.
Your GPH provider will contact you as soon as you are eligible to receive your vaccine. Please note that this is dependent on vaccine allocation and availability.
What is the Moderna COVID-19 vaccine?
The Moderna COVID-19 vaccine is a vaccine that may prevent COVID-19. The FDA has authorized the emergency use of this vaccine to prevent COVID-19 in people 18 years of age and older.
In an ongoing clinical trial, the Moderna vaccine has been shown to prevent COVID-19 following two doses. The Moderna vaccine was shown to be 94.1% effective at preventing laboratory-confirmed COVID-19 in people who received two doses and who had not been previously infected. The vaccine appeared to have high effectiveness in clinical trials among people of diverse age, sex, race, and ethnicity categories as well as persons with underlying medical conditions.
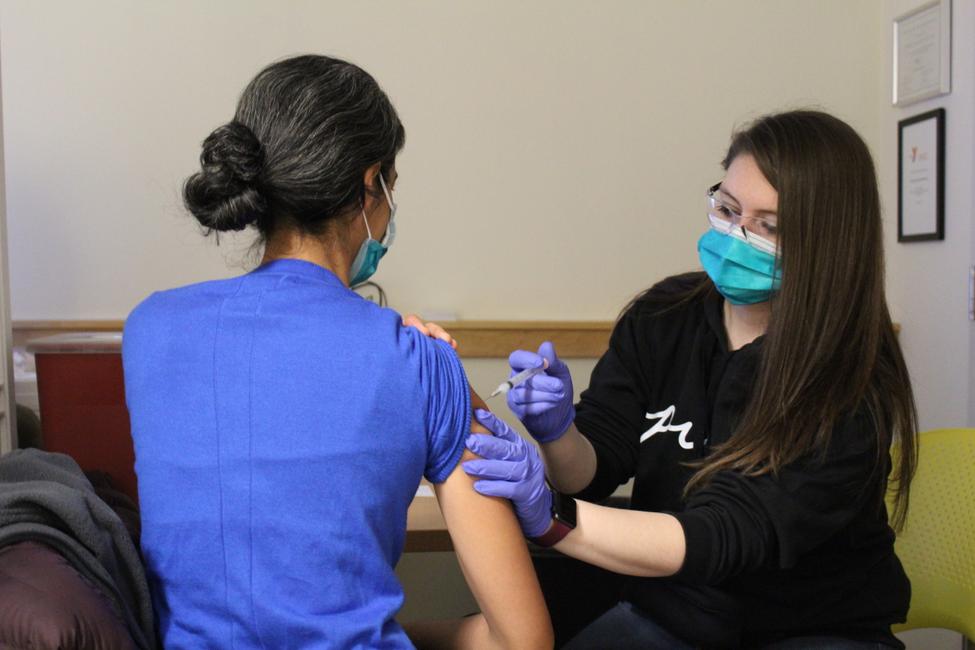
How is the vaccine given?
The Moderna COVID-19 vaccine is given as an injection into muscle. The vaccine is administered in a series of 2 doses given 1 month apart. We are also asking that patients stay with us for a 15-minute observation period after receiving their vaccine.
What are the risks?
Some side effects have been reported with the Moderna COVID-19 vaccine. These include:
- Injection site: pain, tenderness, swelling, redness, and swelling of the lymph nodes in the same arm of the injection
- General effects: Fatigue, headache, muscle pain, joint pain, chills, fever, and nausea and vomiting.
There is a small chance that the Moderna COVID-19 vaccine may cause a severe allergic reaction. While uncommon, a severe reaction will typically occur within a few minutes to an hour after receiving a dose. For this reason, patients are asked to stay for at least 15 minutes of observation.
What is the Johnson & Johnson Janssen COVID-19 vaccine?
The Johnson & Johnson Janssen COVID-19 vaccine is a viral vector vaccine that is recommended in individuals over the age of 18. According to the CDC, this type of vaccine uses a modified and harmless form virus of to deliver genetic instructions to our cells, which helps our immune systems better recognize and respond to COVID-19.
In clinical trials, the J&J vaccine was found to be 66.3% effective at preventing laboratory-confirmed COVID-19 illness in participants who had no signs of previous infection two weeks after receiving the vaccine. These trials suggest that the vaccine has thus far been very effective in preventing serious illness and hospitalization in those who contracted COVID post-vaccination. Early evidence also indicates that the J&J vaccine could help prevent the asymptomatic spread of COVID-19 in vaccinated individuals.
How is the vaccine given?
The J&J COVID-19 vaccine is administered as an injection into the muscle of the upper arm. Unlike Moderna and Pfizer, the J&J vaccine is given only once. For the health and safety of our patients, we are asking that individuals stay with us for a 15-minute observation period after receiving their vaccine.
What are the risks?
Some side effects have been reported with the J&J COVID-19 vaccine. These usually occur within the first 7 days of vaccination and are most common in those between the ages of 18 and 59. Possible side effects include:
- Injection site: Pain, redness, and swelling at the injection site
- General effects: Tiredness, headache, muscle pain, chills, fever, and nausea
There is a small chance that the J&J COVID-19 vaccine may cause a severe allergic reaction. While uncommon, a severe reaction will typically occur within a few minutes to an hour after receiving a dose. For this reason, patients are asked to stay for at least 15 minutes of observation.
For information regarding COVID-19 illness, please see our page: Coronavirus Info
References:
RESOURCES
- COVID-19 Vaccination in Maine - Also available in: Arabic, French, Khmer, Kinyarwanda, Portuguese, Somali, Spanish and more!
- WATCH: Dr. Nirav Shah of Maine CDC speaks to Amjambo Africa about COVID-19 vaccine safety
- WATCH: You Should Get a COVID Vaccine
- COVID-19 Vaccine FAQs
- COVID-19 Vaccine information in 30 different languages
- Portland Public Health; 70+ COVID-19 Information
- COVID-19 Vaccine Sites in Maine
- Arabic
- French
- Khmer
- Portuguese
- Spanish
- Vietnamese